# atoms (mass number of specific element) / (6.02 x 1023)
- Convert Grams To Atoms Worksheet
- Atoms To Grams Conversion Practice
- Atoms Per Gram Calculator
- Atoms To Grams Conversion Formula
- Conversion Of Atoms To Grams
- Calculate Mass In Grams
- Atoms To Mass In Grams Converter Ounces Calculator
Grams of mercury to Atoms of mercury?
For chemistry you often need to convert moles to grams and grams to moles. There is a simple relation between these two:, where - mass of the substance in grams - quantity of the substance in moles - molar mass of the substance in grams/mole. And the most difficult task here is finding out the molar mass.
Converting Grams into Atoms You must first convert grams into moles, then you can convert into atoms. Converting grams into moles: Grams of Mercury / 200.59 (Mass of 1 Mole of Mercury) Converting moles into atoms: Answer to the above * 6.02 x 1023 (Avogadro's Constant) Avogadro's Constant / Mole = 6.022 x 1023 Atomic Mass of Mercury = 200.59
# atoms (mass number of specific element) / (6.02 x 1023). In baking it is a little bit tricky. When a recipe calls for 1 cup of flour, that's a measure of volume, 1 cup being 8 fluid ounces. 'How to Convert Atoms to Grams With a Calculator.' Sciencing, https. How to Find the Mass Number of Bromine With 46 Neutrons. How to Convert AMU to Mole. Convert among weight (mass) units. Convert to grams, miligrams, kilograms, metric tons, ounces, pounds, and tons. Convert among weight (mass) units. Convert to grams, miligrams, kilograms, metric tons, ounces, pounds, and tons. This online conversion calculator will convert among different units of weight or mass. Convert 45 Grams to Ounces How much does 45 grams weigh in ounces? 45 g to oz conversion. From Grains Grams Kilograms Long Tons Metric Tons Milligrams Ounces Pennyweight Pounds Stone Tons Troy Ounces Troy Pounds. (grams) x (6.02 x 10^23) / (mass number of specific element in grams). In order to find the number of atoms in a molecule you must convertthe molecules mass to molar mass, then convert to number. Convert weight and mass culinary measuring units between ounce (oz) and grams (g) but in the other direction from grams into ounces also as per weight and mass units. Culinary arts school: weight and mass units converter. This online culinary weight and mass measures converter, from oz into g units, is a handy tool not only for experienced certified professionals in food businesses.
What is the formula for converting pounds into grams?
There are about 453.6 grams in each pound. So, use this formula: pounds x 453.6 = grams
Converting ounces to grams?
What is the formula for converting grams to dwt?
Converting grams to atoms?
grams of something ( 1 mole something/mass of something)(6.022 X 10^23/1 mole something) = atoms of something
What is the formula for converting ml to grams?
This is not a valid conversion; milligrams (mg) and grams (g) are measures of weight or mass and mL (milliliters) is a measure of volume.
How many atoms are in 1 gram of hydrogen?
Atomic mass- 1.01 Formula- grams × (6.02 × 1023) / atomic mass = number of atoms 1.00 grams H × (6.02 × 1023) / 1.01 grams = 5.96 × 1023 atoms of hydrogen
How atoms of Hydrogen are there in 18 g of water?
Atomic mass hydrogen = 1.01 Formula- grams × (6.02 × 1023) / atomic mass = number of atoms 18 grams H × (6.02 × 1023) / 1.01 grams = 107.29 × 1023 atoms of hydrogen
How many atoms are in 5.00 grams of CO2?
By converting the mass into the number of moles (moles = mass(g)/molecular weight) and then multiplying by avogadro's number you get an answer of 6.842 x 1022 atoms.
What has more atoms 2.88 grams of helium atoms or 16.4 grams of zinc atoms?
2,88 grams of helium have 3,761.1023 atoms; 16,4 grams of zinc have 1,511.1023 atoms.
How many teaspoons in 8.8 grams of dry powder?
There is no real universal formula for converting grams to teaspoons that will work for all ingredients. but it's around 1.88 teaspoons 2 teaspoons ≈ 10g
How many moles of atoms in 3 grams of carbon?
Atomic mass of carbon: 12.0 Formula of grams ---> moles: grams / atomic mass = moles 3.00 grams Carbon / 12.0 grams = .25 moles Carbon
How many moles of atoms are in 7.00 grams of Carbon 13?
0.54 moles of atoms are in 7.00 grams of Carbon 13. The formula used to calculate the number of moles of atoms is No. of moles = Mass divided by Relative tomic Mass or 7 divided by 13.
What is the amount of sodium atoms in 0.75 moles of sodium?
The formula for converting moles to atoms is number of moles × Avogadro's constant Avogadro's constant = 6.022 × 1023 0.75 mole Na × (6.022 × 1023) = 4.517 × 1023 atoms Na
How many ounces in 12 grams?
0.42328754 or 0.5 when rounding up! 12 grams is equal to 0.4232875434 oz (Formula for converting) 12 g* 1 oz 28.34952313 g = 0.4232875434 oz
How do you convert mass into moles of an element?
# of moles = Mass÷ Formula weight Example: 6 grams of Carbon atoms Carbon has an atomic mass of 12 grams so according to the Equation # of moles = 6÷ 12 = 0.5 moles For a compound such as CO2 , Formula weight = ( 1 mole of carbon atom weighs 12 grams + 2 moles of oxygen atoms weighs 32 grams ) 44 grams. Example: 24 grams of carbon dioxide = 24÷ 44…
What would you multiply atoms of germanium by to get the units grams of germanium?
Convert Grams To Atoms Worksheet
There is no direct relationship between atoms of germanium and grams of germanium. Use Avogadro's number to convert atoms to moles, and the atomic mass to convert moles to grams. Since you are converting from atoms Ge, this goes in the denominator (on the bottom) of the first factor. You want to end up in units of grams of Ge, so this goes in the numerator (on the top) of the last factor. atoms Ge1.00…
What would you multiply atoms of boron by to get the units grams of boron?
There is no direct relationship between atoms of boron and grams of boron. Use Avogadro's number to convert atoms to moles, and the atomic mass to convert moles to grams. Since you are converting from atoms B, this goes in the denominator (on the bottom) of the first factor. You want to end up in units of grams of B, so this goes in the numerator (on the top) of the last factor. atoms B1.00…
What would you multiply grams of oxygen by to get the units atoms of oxygen?
There is no direct relationship between grams of oxygen and atoms of oxygen. Use the atomic mass to convert grams to moles and Avogadro's number to convert moles to atoms. Since you are converting from grams O, this goes in the denominator (on the bottom) of the first factor. You want to end up in units of atoms of O, so this goes in the numerator (on the top) of the last factor. g O1.00…
How many atoms of C4H10CL4 will form is you start with 10.00 grams of CL2 and 1.000 grams of C4H10?
The most likely answer is none, because C4H10 is the formula of a saturated hydrocarbon, and such a compound normally reacts with chlorine only by substituting chlorine atoms for some of the hydrogen atoms initially present in the saturated hydrocarbon. Therefore, if four of the hydrogen atoms in each molecule of C4H10 are replaced, the formula of the product would be C4H6Cl4, not C4H10Cl4.
What is th difference between weight and mass?
Mass is the number of atoms in something. Mass is calculated by taking the gram formula mass of the atoms in something (measured in atomic mass units) and converting it into grams and Kg. Weight is a force measured in newtons. weight= mass x gravity, gravity on earth is roughly 10 (9.81) so mass 5kg = weight 50 newtons.
Is 375 grams greater or less than 1 kilogram and explain how you know?
375 grams is less than 1 kilogram. This can be calculated by converting 375 grams to 0.375 kilograms, or by converting 1 kilogram to 1000 grams.
Converting pounds to grams?
If you have 1.8 x 10 to the 23 atoms of copper how many moles of copper do you have?
The formula for converting is: atoms ÷ Avogadro's constant = # moles (1.8 × 1023) ÷ (6.02 × 1023)= 0.299 moles Cu
How many atoms are in 43.5 grams of potassium sulfate?
The formula of potassium sulfate is K2SO4, and the gram formula unit mass is 174.25. The formula shows that each formula unit contains 7 total atoms, and a full gram formula unit always contains Avogadro's Number of formula units. Therefore, the answer is 7(43.5/174.25)(6.022 X 1023) or about 1.50 X 1024 atoms, to the justified number of significant digits.
How do you go from atoms to grams?
# atoms x (atomic mass of element in grams) / (6.02 x 1023) = # grams
How many atoms of Mercury in 65 grams of Mercury?
Atomic mass of Hg: 200.1 grams 65.0 grams × (6.02 × 1023 atoms) / (200.1 grams) = 2.00 × 1023 atoms Hg
How many atoms of gold are in 5 grams of gold?
Atomic mass of Ag: 107.9 grams 5.00 grams × (6.02 × 1023 atoms) / (107.9 grams) = 2.79 × 1022 atoms Ag
What is the formula for converting grams into ounces?
The conversion factor is .035. So, multiply grams by .035 to get ounces. Algebraic Steps / Dimensional Analysis Formula ____ g*1 kg 1000 g*2.2046 lb 1 kg*16 oz 1 lb=? oz Direct Conversion Formula ____ g*1 oz 28.34952313 g=? oz
Convert 20 grams to tablespoons?
There is no real universal formula for converting grams to teaspoons that will work for all ingredients. This is because grams measure weight and teaspoons measure volume. A teaspoon of sugar weight 4.2 grams while a teaspoon of salt weighs 6 grams. A teaspoon of dried parsley only weighs 0.5 gram.
What is the amount of sodium atoms in 0.5 moles of sodium?
The formula for converting moles to atoms is number of moles × Avogadro's constant Avogadro's constant = 6.02 × 1023 0.5 mole Na × (6.02 × 1023) = 3.01 × 1023 atoms Na
18 grams equals how many ounces?
ounces ( avoirdupois) = 18 / 28.349523 = 0.635 ounces (troy) = 18 / 31.103481 = 0.579 The formula for converting grams into ounces:1 g* 1 oz 28.34952313 g = 0.03527396195 oz
How many atoms are in 155 grams of calcium?
Which requires more energy converting 50 grams of ice at 0C to 50 grams of water at 100C or converting 50 grams of water at 100C to 50 grams of steam at 100C?
more energy (latent heat) is required to convert water to steam
What is the mass of 1 mol of nitrogen in grams?
Since the normal formula for elemental nitrogen is N2, this answer is twice the atomic mass of nitrogen or 28 grams. The mass of a mole of nitrogen atoms is half this.
Which has the greatest number of atoms- 3.5 grams of helium or 3.5 grams of lithium?
Helium because it has fewer atoms, so there are more atoms needed to have 3.5 grams of it.
Convert from moles to grams?
For converting any substance in moles to grams:- Grams= No. of moles x Atomic or molecular mass.
Converting 852 g to kg?
There are 1000 grams in a kilogram. 852 grams is 0.852 kilograms.
Converting kilograms to grams?
How to convert grams to atoms?
To convert grams into atoms, you have to convert them into moles first. Get the molar mass and multiply it by the number of moles to get the atoms.
What is the mass in grams of 2x1012 atoms of potassium?
What is the mass, in grams, of 2 × 1012 atoms of potassium?
How many atoms are in 25.1 grams in sulfur?
How many atoms are in 1.9 g Carbon?
Atomic mass of carbon: 12.0 grams 1.90 grams × (6.02 × 1023 atoms) / (12.0 grams) = 9.53 × 1022 atoms C
What is the result of converting 4.3 kilograms into grams?
1 kilogram = 1,000 grams 4 kilograms = 4,000 grams 4.3 kilograms = 4,300 grams
Explain what you can tell about a compound with the formula C6 H12 O6?
From the formula C6H12O6, it is possible to tell that the compound Has six carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms in every one of its molecules Has a molar mass of 6(12.00g) + 12(1.01g) + 6 (16.00g) = 180.12 grams per mol. Has an empirical formula of CH2O (which is not to be confused with the molecular formula, for CH2O is the molecular as well as the empirical formula of formaldehyde, but…
What is the chemical formula for diphosphorus trisulfide?
Diphosphorus trisulfide is comprised of 2 phosphorus (P) and 3 sulfur atoms. its molecular formula is this P2S3 and it has a molecular mass of 148-grams per mole.
How can you fine the mass of mole of atoms of atoms of an element in grams?
Simple: the atomic weight expressed in grams. Example for dysprosium - 162,500 grams.
The mass in grams of 2.72x1021 atoms of Li?
what is the mass in grams of 3.75*10^21 atoms of Li
What is the mass in grams for 25 atoms W?
How many atoms are found in 600 grams of iron?
(grams) x (6.02 x 10^23) / (mass number of specific element ingrams)
How do you convert atoms to grams with Avogadro's number?
divide the number of atoms by avogadros number (6.022*10^23), the resulting number is the number of moles you have. Multiply the number of moles of atoms by the molar mass (found on any periodic table) and the answer is how many grams of the substance you have.
How many atoms are there in 55.8 grams of Fe?
Avogadros number (approximately). The atomic weight of iron is 55.845. Avogadros number , the number of atoms in a mole of an element, or the number of molecules in a mole of a compound is 6.023 X 1023
How to convert grams to atoms?
To convert grams into atoms, you have to convert them into moles first. Get the molar mass and multiply it by the number of moles to get the atoms.
How do you convert from moles to atoms and from mass to atoms?
moles to atoms you multiply the number of moles by avogadros number ex: 1.32 mol x (6.022 x 10^23 atoms)/mol mass to atoms you multiply the mass (in grams) times the molar mass of the element or compound (ex: N 14.01 mols/gram) then times avogadros number once you have the moles. ex: 45.6 g N x (14.01 mol/gram) x (6.022 x 10 ^23 atoms/mol) if it's a compound instead of an element, find the molar…
What is the mass in grams of a single atom of calcium?
You can work this out by dividing the molecular mass of calcium, 40, by Avogadros number, 6.02214129(27)×1023 mol−1 This will give you the answer as the molecular mass of an element is the combined weight of the number of atoms that make up one mole, which is the same as Avogadros number.
Avogadros number of phosphorus P atoms would have a mass of?
By saying Avogadro's number of atoms, you are saying one mole (or 6.02 × 1023 atoms). And one mole of any elements is its atomic mass. Phosphorus' atomic mass is 31.0 grams
How do you convert grams to moles using avogadros number?
You don't need to use Avogadro's number, you need the mass of the molecule in atomic units. Mass / molecular mass = moles eg for water, 36 grams / 18 atomic units = 2 moles
What relationships are needed to convert 1.5 grams of carbon to the number of carbon atoms?
How do you convert moles of atoms to grams?
Multiply number of moles by the molecular mass of the element or compound
One mole is equal to how many grams?
How many atoms are in one gram of calcium?
To work this out you need to know the atomic weight of calcium and Avogadros number, which is the number of atoms in a gram atom. Calcium has an atomic weight of 40.078. Therefore a gram ato is 40.078 grams which contains 6.02214129(27)×1023 atoms. 1g of calcium contains 6.02214129(27)×1023 / 40.078 1.502 X 1022
How do you convert from mass to atoms?
To convert mass to atoms: Find the atomic mass of the element in the substance. You can find atomic masses on the periodic table. Ex. Lithium's atomic mass is 6.9 grams (round if you need to) Then find the mass of the substance in grams. Ex. you have 18.2 grams of a sample of Lithium. The mass of the sample is multiplied by 6.02 * 1023 and divided by the atomic mass. Ex. mass of…
Grams of mercury to Atoms of mercury?
Converting Grams into Atoms You must first convert grams into moles, then you can convert into atoms. Converting grams into moles: Grams of Mercury / 200.59 (Mass of 1 Mole of Mercury) Converting moles into atoms: Answer to the above * 6.02 x 1023 (Avogadro's Constant) Avogadro's Constant / Mole = 6.022 x 1023 Atomic Mass of Mercury = 200.59
Convert 3.55 grams of potassium to atoms?
Firstly, you'll need to convert the grams into number of mols. Since no. of mol = mass/molecular weight 3.55/39.1 = 0.09079 mol because 1 mol of potassium consists of 6.02x10^23 atoms; 0.09079 mol of potassium will consist of 0.09079x(6.02x10^23)=5.466x10^22 atoms of potassium.
What is the total number of atoms contained in 80 grams of neon?
The first step is to identify what you have and what you need. The units of our data is grams. The units that we desire is number of atoms. Therefor, to answer this question, we need two important tools of chemistry: the periodic table, where we can find the molar mass of the element (bottom of the element's box), and Avogadro's number, which is the number of atoms in one mole. Going to our periodic…
How many carbon atoms in a hope diamond of 44.4 carrots?
A carrot? Well I'll assume you mean carat. A carat is unit of mass used by the diamond trade. 1 carat is 200mg. 44.4 carats is therefore 8.8 grams Diamonds are pure carbon. Avogadros number tells us ho many atoms are in a gram atom of carbon this is 6.023x1023 atoms in 12.011 grams of carbon therefore there are (6.023/12.011) X 8.8 X 1023 atoms I make that 4.41 x 1023 atoms
How do you convert milligrams to grams?
To convert milligrams to grams: 1 gram = 1000 milligrams So take the number of milligrams and divide by 1000 to get the number of grams. To convert grams to milligrams: Take the number of grams and multiply by 1000 to get the number of milligrams. Examples: 500 milligrams = 0.5 grams 2 grams = 2000 milligrams
What is the mass in grams of 2.01 x 1022 atoms of sulfur?
1 mole S atoms = 32.065 g S (atomic weight in grams) 1 mole S atoms = 6.022 x 1023 atoms S (Avogadro's number) Convert atoms to moles. 2.01 x 1022 atoms S x (1mol S/6.022 x 1023 atoms S) = 0.0334mol S Convert moles to mass in grams. 0.334mol S x (32.065g S/1mol S) = 10.7g S
How do you convert 2.2 grams in to atoms in nitrogen?
14 grams of nitrogen have 6.023 x 1023 atoms So 2.2 grams will have (6.023 x 1023 x 2.2)/14 = 29.15 x 1023 atoms
What is the mass of 1.505 x 10²³ carbon atoms?
To convert atoms to grams, you need to take the number of atoms, divide it by Avogadro's Constant, then multiply it by the atomic mass. Atoms ÷ (6.02 × 1023) × Atomic mass = Mass in grams 1.505 × 1023 ÷ (6.02 × 1023) × 12.0 = 3.00 grams Carbon
How do you convert from grams to atoms and from mass to atoms?
1 atomgram of a chemical element has 6,02214129(27)×1023 atoms. 1 atomgram=atomic weight of a chemical element exprimed in grams.
The total number of calcium atoms in 80.0 grams of calcium is?
The total number of calcium atoms in 80,0 grams of calcium is 12,044 280.10e23.
The total number of sodium atoms in 46.0 grams of sodium is?
The total number of sodium atoms in 46,0 grams of sodium is 12,044 280.10e23.
How many Cs atoms are there in a 53.7 gram sample of Cs?
This is a two-step calculation. First convert grams Cs to moles Cs. Then convert moles Cs to atoms Cs. The atomic weight of cesium is 133 g/mol 1. To convert grams to moles: moles Cs= 53.7 g Cs1 mol = 0.404 mol Cs133 g Multiply by moles per gram. Grams cancel out. 2. To convert moles to atoms: atoms Cs = 0.404 mol Cs6.02 x 1023 atoms = 2.43E+23 atoms Csmol Multiply by atoms/mole. Moles…
How do you convert 21 grams to Mlli grams?
Multiply the number of grams by 1,000 to convert to milligrams, so 21 grams equals 21,000 milligrams.
What number of Fe atoms and what amount moles of Fe atoms are in 500.0 g of iron?
To convert grams to moles you use the molar mass of iron (55.8 grams per mole). Divide the numbers to get the number of moles: 500 g Fe / 55.8 g/mol = 8.96 mol Fe. To calculate the number of atoms, use Avogadro's Number (6.02*1023). Multiply the number of moles and Avogadro's number: 8.96 mol * (6.02*1023) = 5.39*1024 Fe atoms.
What is the formula to convert kilograms to grams?
Number of grams = (1,000) times (number of kilograms).
How many atoms are in 1.500 kilograms of gold?
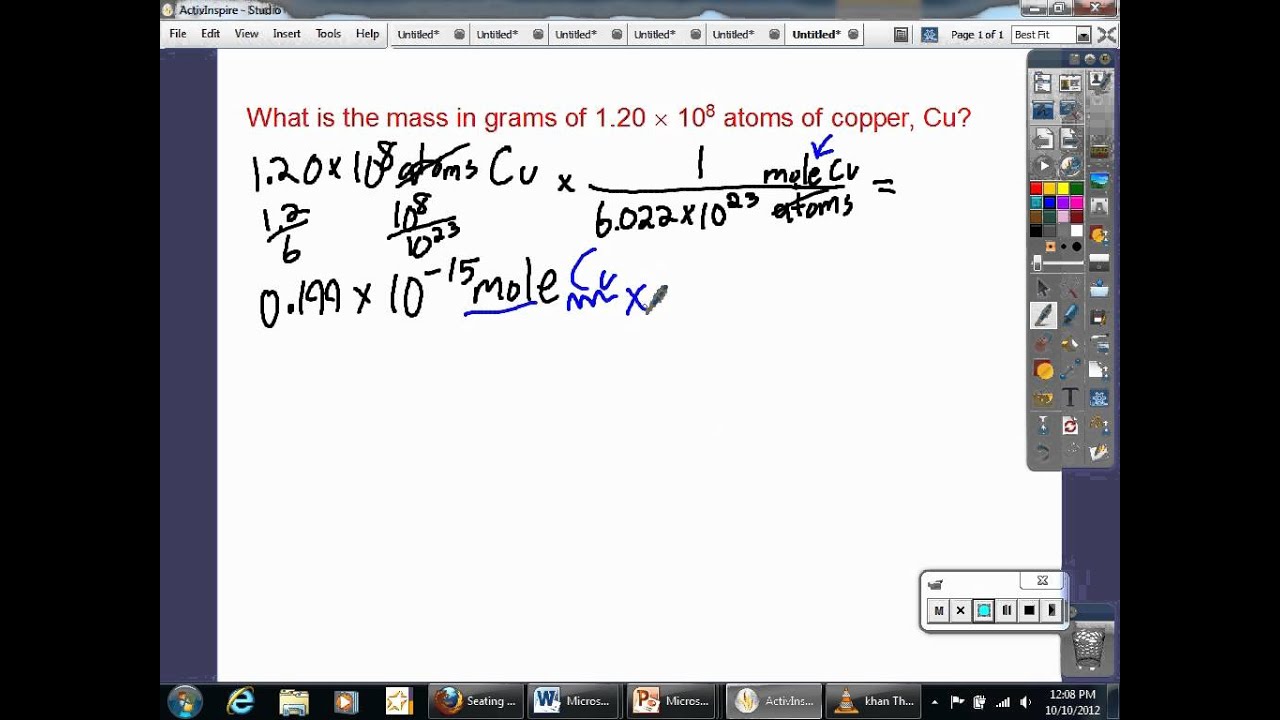
The mass of 1 mole of an element is its atomic weight in grams. 1 mole of an element is 6.022 x 1023 atoms of that element. Known/Given: 1 mol Au = 196.96655g Au (atomic weight in grams) 1 mol Au = 6.022 x 1023 atoms Au (Avagadro's number) 1000g = 1 kg Convert kilograms to grams. 1.500kg Au x (1000g/1kg) = 1500g Au Convert grams to moles. 1500gAu x (1mol Au/196.96655g Au) = 7.616mol…
What would you multiply atoms of germanium by to get the units grams of germanium?
There is no direct relationship between atoms of germanium and grams of germanium. Use Avogadro's number to convert atoms to moles, and the atomic mass to convert moles to grams. Since you are converting from atoms Ge, this goes in the denominator (on the bottom) of the first factor. You want to end up in units of grams of Ge, so this goes in the numerator (on the top) of the last factor. atoms Ge1.00…
What would you multiply atoms of boron by to get the units grams of boron?
There is no direct relationship between atoms of boron and grams of boron. Use Avogadro's number to convert atoms to moles, and the atomic mass to convert moles to grams. Since you are converting from atoms B, this goes in the denominator (on the bottom) of the first factor. You want to end up in units of grams of B, so this goes in the numerator (on the top) of the last factor. atoms B1.00…
What would you multiply grams of oxygen by to get the units atoms of oxygen?
There is no direct relationship between grams of oxygen and atoms of oxygen. Use the atomic mass to convert grams to moles and Avogadro's number to convert moles to atoms. Since you are converting from grams O, this goes in the denominator (on the bottom) of the first factor. You want to end up in units of atoms of O, so this goes in the numerator (on the top) of the last factor. g O1.00…
How many atoms are in 9.0 grams of aluminum?
Avogadros constant is 6.022E+23/mol (number of atoms or molecules in 1 mol of any substance). Standard atomic weight of Al is 26.98 g, so we have 26.98 g/mol. Hence: (26.98 g) / (9 g) = (6.022E+23 / mol) / (x / mol) x = 2.009E+23
Which has the greatest number of atoms- 3.5 grams of helium or 3.5 grams of lithium?
Helium because it has fewer atoms, so there are more atoms needed to have 3.5 grams of it.
How do you convert grams to killograms?
1 kilogram = 1,000 grams. Divide the number of grams by 1,000 to get the number of kilograms.
How do you convert the mass of a substance to the number of moles in a substance?
You must first calculate the molar mass of the substance. To do so, you add up the molar masses of all the elements that make up the compound, multiplied by the number of atoms of that element in one molecule of the substance. For example, AgNO3 has a molar mass of about 169.8 amu. In one molecule of AgNo3, there is one atom of Silver (molar mass 107.8), one atom of Nitrogen (molar mass 14)…
How do you find the mass of a sample of 1.72 x 1023 atoms of potassium?
1 mole K atoms = 39.0983g K (atomic weight in grams) 1 mole K atoms = 6.022 x 1023 atoms K (Avogadro's number) Convert known atoms to moles. 1.72 x 1023 atoms K x (1mol K/6.022 x 1023 atoms K) = 0.286mol K Convert moles to mass in grams. 0.286mol K x (39.0983g K/1mol K) = 11.2g K
How many grams of H2O will contain a total number of atoms equal to Avagadro's number?
Each molecule of H2O contains three atoms. Avogadro's number of water molecules constitutes about 18 grams; therefore one-third of this mass, or 6 grams, will contain the stated number of atoms.
How can you convert grams to kilograms?
Divide the number of grams by 1,000. Then you have the number of kilograms in the same mass.

Conversion grams to pounds?
There are 453.6 grams in a pound. To convert grams to pounds, divide the number of grams by 453.6. That figure will be the number of pounds.
Number of moles and number of atoms in a 214 gram sample of silver?
A gram to mole conversion needs to have an atomic mass. Silver's atomic mass is 107.9 grams. You take the number of grams, multiply it by the number of moles (one in this case) and divide it by the atomic mass. 214 g Ag / (107.9 g) = 1.98 moles Ag Then you convert it to number of atoms. You can skip a step when converting grams to atoms if you have already calculated the…
How do you convert mass to grams?
Mass is measured in grams. If the mass is given in Imperial units such as pounds, convert to grams by multiplying the number of pounds by 453.592.
How many atoms of sulfur are in 10 grams of h2so4?
The way to work this is out is to first find the mass of one mole of H2SO4 .(98.08 grams). 10 grams is therefore 10/98.08 moles- = 0.102 moles of sulfuric acid Each mole of sulfuric acid contains gram atom of sulfur so we have .102 moles sulfur.. if you want the actual count of atoms multiply by avogadros number which is 6.0221415 × 1023 (most teachers will accept using 6.022 instead of the longer…
How many grams are there in 2.3 x 1024 atoms of silver?
Atoms To Grams Conversion Practice
(2.3 x 1024 atoms Ag) x (1 mole Ag/ 6.022 x 1023 atoms Ag) x (107.0 g Ag/ 1 mole Ag) = 412.1056 g Ag Convert the atoms Ag to moles of silver. Then convert the moles to grams.
How do you convert 615 mg to grams?
Divide the number of milligrams be 1000 to convert to grams: 615 mg = 0.615 g
Atoms Per Gram Calculator
What number of Fe atoms and what amount moles of Fe atoms are in 600.0 g of iron?
Known/Given: 1 mole of Fe = 55.845g Fe (its atomic weight in grams) 1 mole of Fe = 6.022 x 1023 atoms Fe (from Avogadro's number) Convert grams Fe to moles Fe. 600.0g Fe x 1mol Fe/55.845g Fe = 10.74mol Fe Convert moles Fe to atoms Fe. 10.74mol Fe x 6.022 x 1023atoms Fe/1mol Fe = 6.468 x 1024atoms Fe
Does 23 g of sodium and 238 g of uranium have equal number of atoms in them?
Atoms To Grams Conversion Formula
dose 23 grams of sodium and 238 grams of uranium have equal number of atoms in them
How many atoms are in 78.79 grams of silver?
The atomic weight of silver is 107.87, so divide 78.79 by 107.87 to get the number of moles of silver in 78.79 grams, which is 0.7304 moles of silver. The number of atoms in one mole of any substance is 6.022 * 1023. So multiply 0.7304 by this number to get the number of atoms in 78.79 grams of silver. 4.399 * 1023 atoms
How many atoms are present in 1 milligram of oxygen?
one mole of oxygen = 16grams and is 6.02x10^23 atoms one milligram of oxygen is 0.001 grams. number of moles = mass used/ RMM of compound therefore number of moles = 0.001/16 =6.25x10^-5 moles used. to convert to number of atoms multiply by avagadros number. 6.25x10^-5 * 6.02x10^23 3.763x10^19 atoms
Conversion Of Atoms To Grams
What is the mass of atoms in grams of 1.02X10 to the 24 atoms Mn?
1 mole manganese = 54.938049g 1 mole Mn atoms = 6.022 x 1023 atoms Convert atoms Mn to moles Mn. 1.02 x 1024 atoms Mn x 1mol/6.022 x 1023 atoms = 1.69mol Mn Convert moles Mn to mass in grams Mn. 1.69mol Mn x 54.938049g/mol = 92.8g
Calculate Mass In Grams
How do you convert grams to atoms?
Atoms To Mass In Grams Converter Ounces Calculator
(grams) x (6.02 x 1023) ÷ (mass number of specified element in grams) Find the mass of the specified element by using the atomic mass on the periodic table. If it is a compound, add up the atomic masses of each atom in the compound.